Precision Oncology Has a Coordination Problem: POV from DeepScribe CEO Matthew Ko
Despite the increasing complexities and personalization of cancer care, organizations use the same systems and workflows to manage it all. Here’s why oncology treatment planning breaks at scale—and why a new operating layer is a must.

By Matthew Ko, DeepScribe CEO and Founder
This article was originally published on LinkedIn in January 2026.
Precision oncology is no longer aspirational. Sequencing is becoming routine. Targeted therapies are widely available. Clinical guidelines are updated continuously as evidence evolves.
Yet, outcomes still lag behind what science makes possible.
This gap is not the result of unsolved biology. It is driven by an operating model that cannot keep up with the complexity it has created. At scale, cancer care is not constrained by knowledge. It’s constrained by coordination.
Nowhere is this more visible than in treatment planning. Clinical evidence, molecular findings, prior decisions, operational constraints, and patient preferences must be synthesized into a plan that is both medically correct and executable in the real world. When coordination fails here, the system does not break loudly. Instead, it does so in ways that are hard to see and even harder to recover from.
Treatment planning: the point of maximum strain
Treatment planning sits within nearly every moving part of cancer care. Diagnostic history, molecular testing, tumor board input, prior lines of therapy, comorbidities, payer rules, drug access, and patient intent all converge at once. This happens under immense time pressure, often with incomplete information, across teams that rarely share a single system or workflow.
Precision medicine demands not just precise medicine but precise execution.
Each plan is inherently bespoke. There are no true templates. Precision medicine demands not just precise medicine but precise execution.
Historically, however, healthcare absorbed this complexity by leaning on clinicians. When information was fragmented, clinicians compensated. When workflows were brittle, clinicians bridged the gaps. When documentation was incomplete, clinicians reconstructed intent from memory, experience, and hallway conversations.
This may have worked when the practice of oncology was simpler. It doesn’t work now.
As therapies become more targeted and guidelines more granular, the system asks clinicians to integrate a deluge of information at a level that exceeds what any individual can reliably sustain. What we are seeing across oncology is not unpredictable failure. It’s coordination debt finally coming due.
Why legacy workflows fail in predictable ways
When coordination depends on human recall, failure becomes systemic rather than episodic.
A 2024 ASCO analysis of more than 38,000 patients with advanced non–small cell lung cancer found that 64% of eligible patients never received the targeted therapy appropriate for their biomarkers. There wasn’t a common step in which failure occurred. Instead, patients fell out across seven operational handoffs: biopsy referral, specimen quality, test ordering, testing, reporting delays, interpretation, and treatment selection.
This wasn’t a story of clinical disagreement or uncertainty. It was one of workflow attrition.
The upper bound of precision oncology is now being set by operational friction, not scientific progress.
A 2025 abstract in Diagnostics reached a similar conclusion. One quarter of medical oncologists were unaware of updated NCCN recommendations to test at metastatic diagnosis.
The guidelines were accessible. But they were evolving faster than existing workflows could reliably surface them at the moment decisions were made.
Across the literature, the pattern repeats:
- Approximately 10 to 35% of eligible patients never receive recommended biomarker testing.
- Approximately 30 to 40% of patients with actionable mutations never receive matched therapy.
The uncomfortable truth is that the upper bound of precision oncology is now being set by operational friction, not scientific progress.
For patients, the impact of missed tests or non-targeted therapy can be staggering. It can mean the difference between months and years of survival.
For health systems, it means unnecessary spending, avoidable complications, and lost clarity in clinical outcomes data.
Memory cannot be infrastructure
Today’s treatment planning process rests on an unscalable assumption: Critical context lives in clinicians’ heads and moves forward effectively through notes, PDFs, inboxes, portals, and verbal handoffs.
Often, that assumption holds. Until it doesn’t.
As molecular diagnostics expand and therapeutic options proliferate, cognitive demand grows far faster than human capacity. The resulting failures are rarely dramatic. They show up as delayed decisions, missed eligibility, inconsistent interpretation; when precision medicine fails, it does so one overlooked detail at a time.
This is why the problem persists even in well-resourced cancer centers. The issue is not that the knowledge doesn’t exist. It’s that it fails to be applied. It’s not the lack of effort. It’s the lack of the right system architecture.
An analogy: From visual flight to air traffic control
There is a useful analogy in aviation.
Early flight relied on Visual Flight Rules (VFR), by which pilots navigated by sight, judgment, and experience. As air traffic increased, this approach failed, but not because pilots’ skills waned. It failed because the system outgrew what individual judgment could safely coordinate.
The solution was not better pilots. It was air traffic control.
Air traffic control did not replace pilot judgment. It created a shared operating layer that maintained situational awareness across the system. It ensured clean handoffs and prevented small coordination failures from compounding into catastrophic ones.
Oncology is now at a similar inflection point. We are still flying visually in a world that now demands coordinated traffic management.
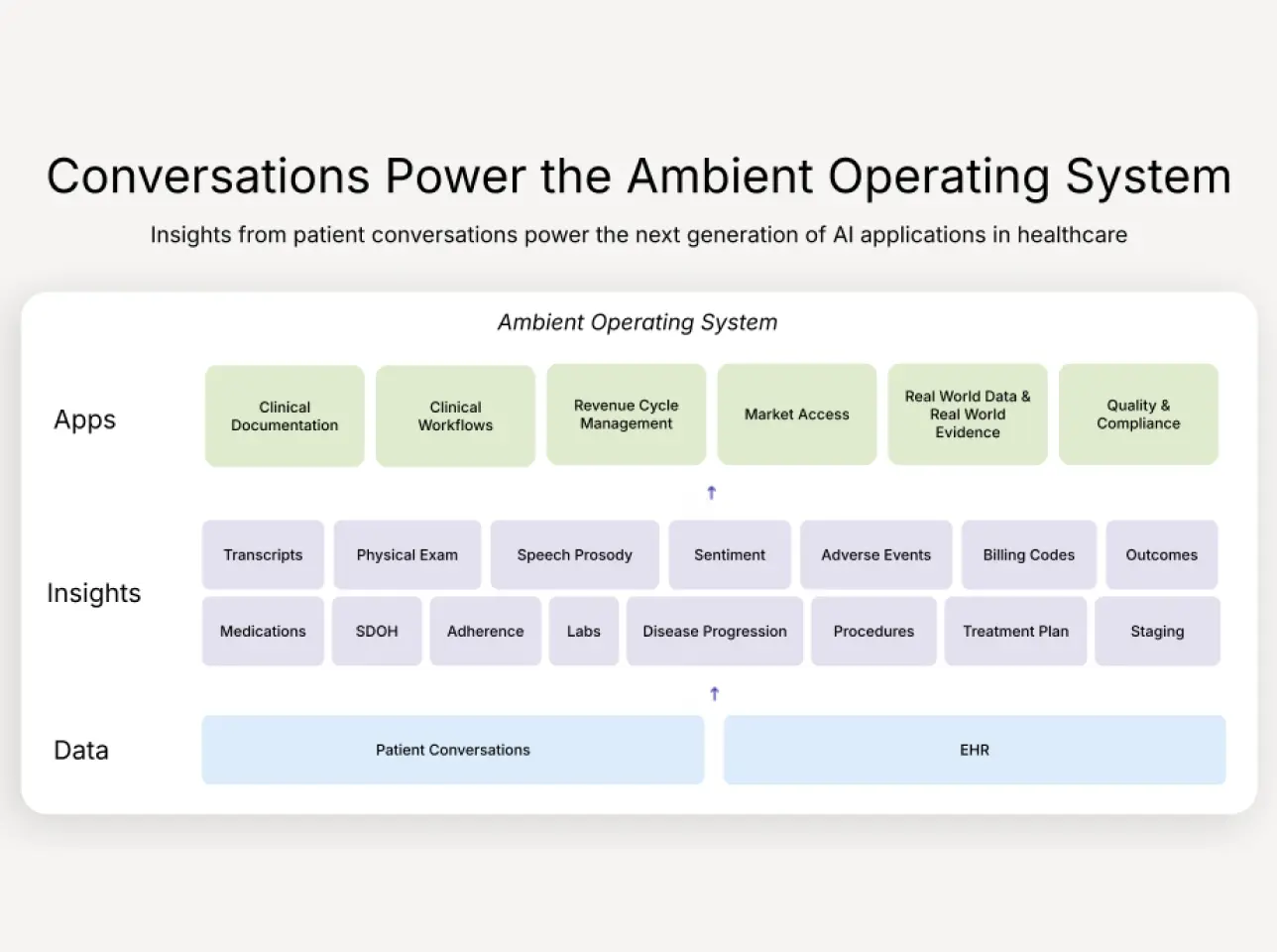
Ambient AI as treatment coordination infrastructure
When applied to treatment planning, ambient systems change the nature of reliability.
Instead of relying on clinicians to recall whether guideline-recommended testing has occurred, systems can surface the most up-to-date testing guidelines at the point of care.
Instead of biomarker results living in PDFs or external portals, they can be normalized and presented in the patient’s clinical context.
Instead of treatment rationale dissipating across tumor board, clinic, pharmacy, and infusion, it can persist as structured intent across the care continuum.
This is not automation for its own sake. It is coordination enforcement.
Ambient systems can identify when a plan is drifting away from current standards. Not after the fact, but while there is still time to intervene... It informs, flags, and creates the conditions for earlier course correction.
The goal is not to choose care or replace judgment. The goal is to ensure that what clinicians decide actually gets executed.
What makes this shift different from prior tooling is that context is no longer trapped inside individual workflows or people’s heads. It becomes shared infrastructure. Intent is captured when it is formed, carried forward across teams, and validated against real clinical and operational constraints.
Over time, this same infrastructure begins to do something more subtle and more powerful. It starts to act as a safety net.
Ambient systems can continuously observe imaging, pathology, molecular data, guidelines, prior decisions, and newly emerging evidence. They can identify when something unexpected has not occurred, when a prerequisite is missing, or when a plan is drifting away from current standards. Not after the fact, but while there is still time to intervene.
Think of this more like radar than an alert. Clinicians remain fully in control. The system maintains situational awareness across the treatment planning process and surfaces potential issues before they become errors. It informs, flags, and creates the conditions for earlier course correction.
That is what allows expert judgment to scale.
Let’s get more specific. Here are some examples of what this coordination infrastructure could look like in the real world—and is just around the corner:
Surfacing incomplete or missing testing before it becomes a treatment delay
One of the most preventable failures in oncology is testing that’s ordered too late, never ordered, or quietly overlooked. These gaps often persist until disease progression exposes them.
As therapies have become more personalized, timely testing is now a prerequisite for effective care.
Nearly 18% of patients did not receive appropriate biomarker testing because no system reliably surfaced the need at the right moment.
Today, identifying testing gaps depends almost entirely on a vigilant clinician manually reviewing the chart. The ASCO analysis highlights the fragility of this approach: Nearly 18% of patients did not receive appropriate biomarker testing, not because testing lacked value, but because no system reliably surfaced the need at the right moment.
Ambient coordination changes this dynamic. Instead of assuming someone will remember, systems can recognize when to order guideline-recommended tests, surface prior results during treatment planning, and flag discrepancies between visit type and available data.
Integrating results and life context at decision-making moments
Once testing results are available, a second major failure mode emerges in how they’re shared. The results are there, but buried or surfaced too late. The problem is that they’re not seamlessly embedded into the clinical workflow.
Ambient AI can surface structured biomarker findings directly into the treatment planning moment, highlight guideline-aligned therapy considerations, and reduce the risk that critical data is overlooked.
Oncologists are forced to synthesize information scattered across EHR fields, unstructured pathology PDFs, scanned documents, and external portals. Results exist, but they are buried, inconsistently interpreted, or surfaced too late.
Ambient AI can surface structured biomarker findings directly into the treatment planning moment, highlight guideline-aligned therapy considerations, and reduce the risk that critical data is overlooked or misread.
Just as important, ambient systems can integrate life context into treatment planning. The details can be overwhelming: age, comorbidities, prior lines of therapy, functional status, transportation challenges, financial constraints, family obligations, and personal milestones all shape what is appropriate.
These details are frequently shared in conversation and then lost somewhere between the visit and the chart, or never structured in a way the system can use. Ambient systems capture these signals in real time, structure them, and bring them forward in context, at the moment decisions are made. With the information where it’s needed, oncologists can more easily plan care around a person’s life, not just their disease.
Preserving treatment rationale across the care continuum
Behind every treatment decision is its rationale, the reasons for choosing a specific therapy and the constraints that shaped the plan.
Today, the use of that rationale is fragile and difficult to access at scale. It may live in tumor board notes, separate documents, or verbal handoffs. It is rarely structured for downstream use and often lost as care transitions from planning to execution.
Ambient systems can capture this reasoning as it is discussed and carry it forward as structured context. That can then be surfaced to pharmacy, infusion, nursing, radiation, and downstream clinicians responsible for execution, in ways that are fit for their use case.
Not only does this integrate treatment rationale into the process with consistency, it also reduces rework, prevents misalignment, and strengthens safety. It is coordination made durable.
Treatment coordination as a lever for equity
Many of the most persistent inequities in oncology emerge early. ASCO and NCI analyses show disparities in biomarker testing and targeted therapy by race, socioeconomic status, geography, and practice setting.
The underlying problem is not intent. It is inconsistency.
There is no reliable workflow ensuring every patient is evaluated the same way. Ambient systems help standardize what should already be standard by surfacing expectations consistently and making relevant context visible—to every clinician, for every patient.
In this framing, equity becomes a property of the system rather than an aspiration layered on top of it.
Why timing matters now for oncology
The organizations that will lead the next era of oncology will recognize that precision medicine is no longer limited by what we know, but by how reliably we execute. They will see ambient AI not as a tool, but as an operating layer. An infrastructure for coordination that sits above fragmented systems and ensures that intent, context, and decisions survive in real-world care.
With ambient systems in place, these organizations can make execution more dependable. Gaps are caught before they become delays. Risks are surfaced before it’s too late. And increasingly personalized care can actually be delivered consistently across clinicians, sites, and patient populations.
Change used to be the biggest risk in healthcare. Now, the greatest risk is piling up complexity without an underlying architecture that allows intelligence to compound rather than fragment.
Those that don’t make this possible will continue layering complexity onto workflows built for a simpler era. In the near term, this often looks like progress: adding AI point solutions to address individual pain points, each helpful in isolation. Over time, however, this approach increases fragmentation. Context becomes further distributed across tools that do not share a common substrate, and the system becomes more dependent on humans to reconcile what machines cannot.
Change used to be the biggest risk in healthcare. Now, the greatest risk is piling up complexity without an underlying architecture that allows intelligence to compound rather than fragment.
Final thoughts: How precision oncology can be truly realized
The ASCO data makes one reality unavoidable: Precision oncology does not fail because the science is lacking. It fails because execution is unreliable.
Every patient deserves the therapy their situation calls for.
Every clinician deserves systems that do not depend on heroics.
Precision medicine only works if the system can execute it.
Ambient AI, properly understood, is about far more than just writing better clinical notes. It’s about building the coordination infrastructure that ensures the right clinician gets the right information for each patient, at the right moment.
This is part of a continuing series exploring oncology’s value chain and the ways ambient AI can meaningfully strengthen the moments of care. For more, read the article “Ambient AI and the Oncology Value Chain: Ways to Reduce the Cost of Complexity.”
Data sources: “Perceived Barriers to NGS-Based Molecular Profiling Among US Metastatic Breast Cancer Patients,” Diagnostics, October 2025; “Strategies to Address the Clinical Practice Gaps Affecting the Implementation of Personalized Medicine in Cancer Care,” JCO Oncology Practice, March 2024
text
Related Stories
Realize the full potential of Healthcare AI with DeepScribe
Explore how DeepScribe’s customizable ambient AI platform can help you save time, improve patient care, and maximize revenue.